HATUA – KENYA
HATUA – ENABLING COMPLIANCE AND BUILDING CAPACITY AND COMMUNITY FOR CLINICAL RESEARCH IN KENYA (HATUA – KENYA)
HATUA is a project funded by European & Developing countries Clinical Trials Partnership (EDCTP). The core institutions engaged in undertaking this project include: the Kenya Medical Research Institute (KEMRI), the National Commission for Science, Technology and Innovation (NACOSTI), the Pharmacy and Poisons Board (PPB), Synergy Informatics, intelliSOFT Consulting and the Council of Health Research for Development (COHRED). In addition, the project will engage local private and public universities and the Bioethics Society of Kenya (BSK).
The HATUA Project focuses on improving the 3 C’s in research namely: Compliance, (improving functions for oversight of clinical trials), Capacity (building technical capacity through training in Bioethics, pharmacovigilance, clinical monitoring and research administration) and Community (build a network for sharing and a community of best practices through seminars conferences and online consultation).
GOALS to be achieved through HATUA
COMPLIANCE
To enable the Kenyan National Ethics and Regulatory bodies to improve
their function, oversight, and communication with the IREC in the conduct of clinical trials.
The following activities have been undertaken to enable the Kenyan National Ethics and Regulatory bodies (NACOSTI & Pharmacy and Poisons Board) and Institutional Research Ethics Committees to improve their function and communication for oversight of Research regulation.
NACOSTI
- Kenya National Research Information system (KENRIS)
NACOSTI’s mandate and strategic plan objectives include the creation of a register for all research institutions and researchers in Kenya. This has been accomplished through the development of an online registration platform and database of all institutions and researchers in Kenya
National Research Information system (KENRIS). The KENRIS was developed by Synergy informatics Ltd which is a locally based firm that provides information and technology (IT) services to the medical industry. The organization develops solutions that support processes in the healthcare sector ranging from medical research to clinical practice.
The system enables tracking of institutions that have accredited IREC and also of researchers who are duly registered in the system. The live version of the RIRS system was launched on 23 rd July 2020 and is available on https://rirs.synrnf.com . This online system can be accessed through Mobile phones or over the web.
Once the system was launched, NACOSTI held a virtual meeting with IERC representatives on the 9th of July 2020 to sensitize them on how to use the RIRS. The participants were instructed on how to register on the system and how to provide information to NACOSTI on their ongoing research activities. In addition, they were informed about how to have seamless data and information exchange between their institutions and NACOSTI.
PPB
- CTR information system and Pharmacovigilance Electronic Records System
At the Pharmacy and Poisons Board (PBB) there are pharmacovigilance reporting mechanisms in place the Clinical Trial Reporting system and Pharmacovigilance Electronic Records System (PVERs) which were important to improve Compliance, increase capacity and Nurture a Community of Practice. However, these systems were not well developed to take care of safety concerns arising from clinical trials (e.g. Severe Adverse Events (SAEs).
Intellisoft consulting, a partner in the HATUA project upgraded the systems to enable setting up of an online system for the reporting of SUSARs, SAEs, and their evaluation to assist in taking the necessary regulatory actions. The upgraded system also allows for inspection of clinical trial sites so as to ensure compliance to protocol and the generation of robust data that is trustworthy. The enhanced CTR & PVERs system was deployed at PPB and an On-job training approach
preferred by PPB was adopted.
PPB staff having been involved throughout the design and development of the CTR & ePV system based on the gaps in their previous workflows, and with access to the test environment to simulate the expected workflows, the PPB team opted for an on-job-training approach. Internal coaching and mentoring were adopted in PPB.
The enhanced system was deployed at PPB in March 2020 and has been in use since. More information about the system can be found on the following website; Click here . The system has been very helpful during the pandemic as PPB has migrated all Clinical trials reports to the online system.
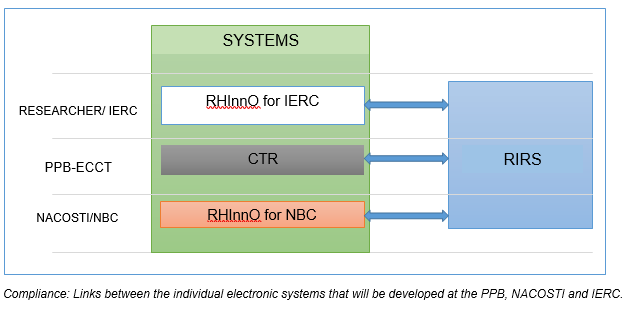
- RHInnO Institutional Installation
To improve efficiency and of accredited IRECs with a high volume of Clinical trials : HATUA has been working with the Council for Health Research and Development (COHRED) to install nine RHInnO Ethics online platform packages (rhinno.net): 8 at the institutional level and one at the national level (NACOSTI), Institutions were selected based on their volume of clinical trials. COHRED’s Research for Health and Innovation Organiser (RHInnO Ethics) is an innovative cloud-based platform or ‘Software as a Service’ (SaaS) enabling Research Ethics Committees (RECs) / Institutional Review Boards (IRBs) in low and middle-income countries (LMICs) to deliver better quality, more efficient, accelerated and standardized ethical review of research for global health. Implementation of RHInnO Ethics drastically reduces the review time by as much as 12 months of study protocols, particularly in complex, multi-center studies that are increasingly becoming the norm to test new medicines, vaccines, technologies, diagnostics, and services.
Through funding from the HATUA Kenya project, COHRED has so far installed the RHInnO system at Gertrude’s Children’s (GCH). Before the system was installed at GCH, nine participants, including five IERC administrators from GCH and two from Kijabe Hospital, were trained on how to use the system. The funding will enable the COHRED team to continue providing technical support for institutions where they will install the system for two years, after which the institutions will be expected to take up financial responsibility for the long-term sustainability of the system beyond the life of the HATUA Kenya Project.
COHRED is in the process of installing the RHInnO system for KEMRI and Moi University
CAPACITY BUILDING
To build technical capacity for the Kenyan National Ethics and Regulatory bodies and IERCs, the HATUA Kenya Project has been supporting capacity building activities through long-term and short-term training. The overall goal is to improve the capacity for review of research, and oversight of clinical trials in Kenya.
Short term training has targeted skills building for the competence of various cadres of staff involved in research regulation activities. This has included training of IERC Administrators and Secretariat to build capacity for this cadre of staff and equip them with the necessary skills they need to increase their efficiency within the committees. This training is done through a certificate course that was developed for and by research administrators who suggested topics they deemed important to be trained in to enable them to carry out their day to day duties in the committees as secretariat/ administrators.
So far we have done two trainings, where we have requested IERCs to select the administrators/secretariat they would like to train.
- Research Administration Pilot Training 2019
HATUA organized a 3-day research administrators pilot training from 31 st July to 2 nd August 2019 at the KEMRI headquarters. This training was developed for and by research administrators and secretariat who serve within Institutional Review Committees (IERCs) in different capacities.
The aim of this pilot training was to build capacity for this cadre of staff and equip them with the necessary skills they need to increase their efficiency.
- Research Administration Training 2020
HATUA project organized a three-day virtual training from 15th to 17th June 2020. Institutional Ethics Review committees nominated administrators from their committees to be trained. A total of 22 representatives were trained virtually.
Long term Training
HATUA project is currently supporting 6 students who are currently enrolled in the online Master’s program at Anahuac University Mexico and Trinity International University, USA. The individuals include:
TIU:
- Ms. Everlyne Ombati-KEMRI-She is the Advocacy and Regulatory Affairs coordinator with the Research Care and Training Programme. She also serves as a member of the KEMRI Scientific and Ethics Review Unit (KEMRI, SERU). She is in her second year of study.
- Mr. Rachier Amollo- KEMRI-ERC-He is the current chair of the Scientific and Ethics Review unit at KEMRI. He is a practicing lawyer and not an employee of KEMRI. He is in his second year of study.
Anahuac University in Mexico commenced in April-2019. All the students are in their second year of study starting in April 2020.
- Ms. Teresia Nyawira- NACOSTI- She works under the regulatory affairs department Ms. Sarah Karanja- AMREF ERC She is the secretary to the ERC.
- Ms. Mercury Shitindo-Strathmore university ERC- She is an administrator of the ERC.
- Mr. Martin Maitima- Mbagathi Hospital ERC- He is a member of the committee.
COMMUNITY
To build a network for sharing experiences and best practices: This will create space for the sharing of best practices and/or challenges. This will include the engagement of a civil society organization such as the Bioethics Society of Kenya (BSK).
The Bioethics Society of Kenya and the HATUA Kenya project signed an MOU to organize annual bioethics conferences, scientific workshops, and bioethics cafes. The target participants have included IERC members and any other institutions and individuals interested in ethics in the life sciences.
In addition, capacity-building workshops have been organized targeting specific identified needs for clinical trials and bioethics cafes targeting institutions of higher learning. The following are the events that HATUA and BSK have organized:
- Bioethics cafes were conducted :
I. Egerton university-6th November 2018
II. Pwani University-2nd November 2018
III. Technical University of Mombasa- 17th October 2018
IV. Kisii University- 31 st May 2019
V. Maseno university Virtual Bioethics café – 6th August 2020.
VI. University of Kabainga Virtual café- 27th October 2020
The composition of participants were mainly students from the College of Health Sciences and their teaching staff (Scientific Community higher education, Research)The cafés focused on the following topics;
i. Bioethics discourse-Principles and Infrastructure in Kenya,
ii. Research ethics in Kenya- From principles to policy
iii. Bioethics and public health ethics in Kenya
iv. Reviewing research ethics in Kenya
v. An Introduction to the Bioethics Society of Kenya
vi. Panel Discussion
vii. Ethics around Pandemics and Epidemics and the Moral Issues around Use of Patients. Clinical Data- This topic was specific to the virtual café in the midst of the COVID-19
pandemic.
viii. Integration of Ethics in Research
ix. Legal and Policy Issues in Research
- Clinical Trial Capacity Building Workshops
HATUA project in collaboration with KEMRI Centre for Global Health Research (CGHR) hosted a one-day clinical trial capacity building workshop in Kisumu. Dr. Edward Abwao, who is the Head of the Clinical Trial Unit at PBB made a presentation on clinical trials regulation in Kenya. The workshop was attended by 31 scientists working in KEMRI CGHR.
In addition, HATUA in collaboration with the KEMRI Scientific Ethics Regulatory Unit (SERU) organized a two-day capacity building workshop that was held on 26th -27th June 2019 at Lukenya. During the workshop, the HATUA project had a session on clinical trials. The participants watched a movie titled WIT which depicts the existential phenomena that occur in times of medical crisis and at the frontier of death. Vivian, a ruthless scholar of 17th Century English poetry, is diagnosed with advanced-stage 4 metastatic ovarian cancer. Dr. Harvey Kelekian, Vivian’s consultant physician and leading figure in this area of medical research, explains that the most effective treatment option is undergoing chemotherapy.
There are a number of ethical issues that are raised in this movie including; the conflict of interest witnessed between clinical therapy and clinical research, entangled with clinical incompetence, and the challenges of informed consent processes which the participants discussed.
- Annual BSK Conference- 2019
HATUA Kenya Project partnered with BSK to organize the 3rd BSK conference, which was held on 15th – 16th 2019 held at the Anglican Church of Kenya Guest House, Nairobi Kenya. The theme of the conference was ‘Contemporary Ethical Challenges’. Representation of participants was from research institutions, private and public universities, hospitals, and individuals interested in bioethics. 52 participants attended the conference.
The conference had seminar presentations from selected keynote speakers and article presentations on selected thematic areas including Ethical Issues in Healthcare, Ethics of Gene, Editing for Reproductive Health and Treatment, Health Insurance, Economics, and Ethics among others.
The conference provided an opportunity for networking and sharing of experiences among participants in their field and best practices. The conference provided a platform for sharing and disseminating information in the field of bioethics through; abstract presentations and presentations on different sub-themes on Contemporary ethical challenges.
- Annual BSK Conference -2020
HATUA Kenya Project partnered with BSK to organize the 4th BSK conference, held on 12th and 13th March 2020 held at the Swiss Lenana Mount Hotel, Nairobi Kenya. The theme of the conference was ‘Contemporary Issues in Bioethics’.
Representation of participants was from research institutions, private and public universities, hospitals, and individuals interested in bioethics. 65 participants attended the conference.
The conference had seminar presentations from selected keynote speakers and abstract presentations on selected thematic areas including; ethical implications in research and use of new health technologies, stem cell research, biobanking concerns, assisted reproductive technologies, sharing benefits and burdens in collaborative research, ethics of artificial intelligence among others.
The conference provided a platform for sharing and disseminating information in the field of bioethics through; abstract presentations on different subthemes on Contemporary Issues in Bioethics. It was also an opportunity for participants to network and share experiences and best practices.
 IntelliSOFT Consulting
IntelliSOFT Consulting
i. Develop the platform that facilitates communication between the IREC and the PPB when clinical trials are approved.
ii. Develop online submission system for adverse events for ongoing clinical trials

Council on Health Research for Development
Will provide technical support to implement the RHInnO Ethics (online based submission and review system) at both theNational Bioethics Committee (NBC) and the IERC levels.

National Commission For Science, Technology & Innovation
Will provide strategic direction for
implementation of the project.
Synergy Informatics Ltd
Will develop an online platform for registration of research institutions and researchers: the Researchers and Institutions Registration System (RIRS)
Bioethics Society of Kenya
Will develop and implement appropriate
workshops targeting those providing administrative support to IERCs and building
capacity for NACOSTI, PPB and the IERC.
CONTACT INFORMATION
Phone: +254737041739
Email: ek.ro.ptcr@autah / moc.liamg@81aynekautah
This project is part of the EDCTP2 Programme supported by the European Union





