
Top KEMRI and WRAIR Leadership Negotiate for a New Collaborative Agreement.
February 26, 2025
KEMRI Bolsters Global Research Collaborations with UK Partners.
April 17, 2025Three Decades of Malaria Research in Kenya: A Story of Breakthroughs and Surprises.
For the last 30 years, the Kenya Medical Research Institute (KEMRI) has been at the forefront of groundbreaking malaria research, significantly improving treatment and prevention strategies that have been recommended by the World Health Organization (WHO) as intervention measures against malaria particularly for vulnerable groups such as pregnant women and young children.
While making the keynote address at the 15th KEMRI Annual Scientific Conference (KASH), Prof. Feiko ter Kuile of Liverpool School of Tropical Medicine (LSTM) and situated at the KEMRI’s Centre for Global Health Research (CGHR) highlighted some of the many key milestones in the fight against malaria resulting from the research at CGHR, and the evolution of its clinical research capacity. From humble beginnings in 1995 with just 50 staff members, the malaria division has expanded to over 450 researchers, producing world-class studies that have shaped global malaria policies with increased first and senior authorship and well over 50% of the PI’s being female.
Other great milestones include moving from 2 to 15 PhD students, increased grant writing and income with Kenyans being the grant holders.
His remarks covered the surprises and breakthroughs of research in malaria in pregnancy and post-discharge malaria chemoprevention for hospitalized children with severe anaemia.
“This is a story of surprises and breakthroughs and the power of partnership,” stated Prof. Feiko.

During pregnancy, malaria is more frequent and more complicated compared to age-matched nonpregnant women. Malaria in pregnancy leads to severe complications, including preterm birth, low birth weight, and increased perinatal mortality. Over 122 million pregnancies are at risk annually, with 46 million of these occurring in Africa.
Between 1996 – 2004, KEMRI researchers pioneered preventive strategies such as the use of insecticide-treated bed nets (ITNs) and Intermittent Preventive Treatment in Pregnancy (IPTp) with the antimalarial sulfadoxinepyrimethamine (SP). Both interventions have been recommended by the WHO as intervention measures against malaria in 2000 and 2004 respectively. To date, these interventions are used all over Africa and have shown remarkable success, reducing maternal anemia by 38%, low birth weight by 43%, and perinatal mortality by 27%. However, the challenge of drug resistance has led researchers to explore alternative prevention strategies, such as screen and treat strategies with rapid diagnostic testing and new antimalarial drug combinations to replace SP.
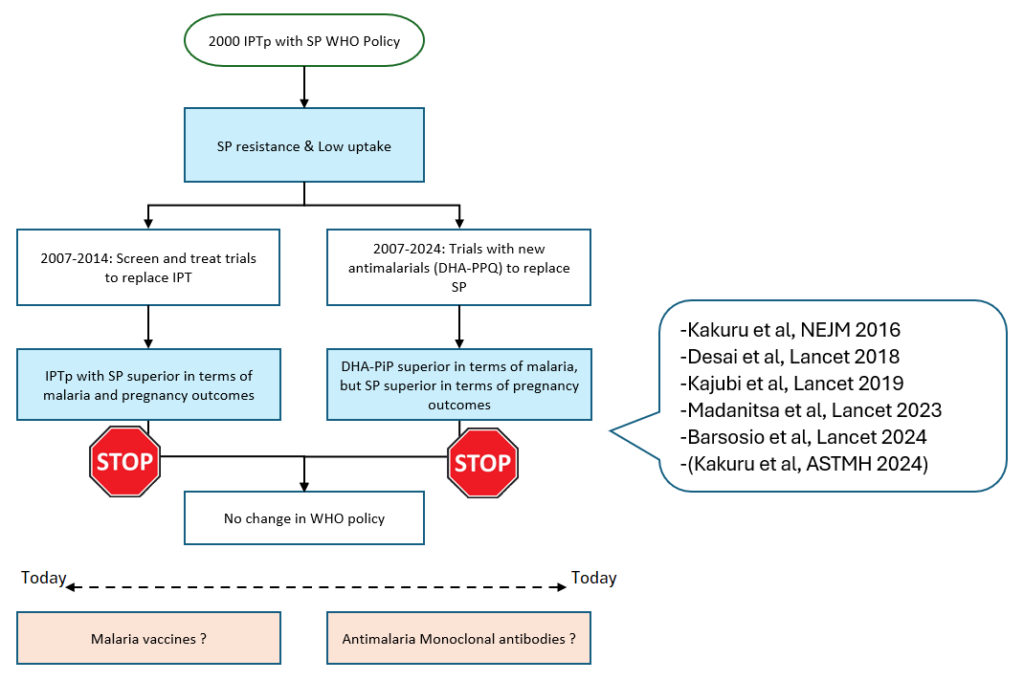
This led to a series of studies conducted all over Africa looking at alternative drugs to replace SP. Most of these existing drugs were very effective but not tolerated well enough by pregnant women. In 2015 however, teams at the KEMRI CGHR and the Infectious Diseases Research Collaboration (IDRC) in Uganda conducted initial proof-ofconcept studies published in the Lancet and New England Journal of Medicine and found that the newer drug DHA– piperaquine (DP) was very well tolerated and much more effective than SP at preventing malaria in pregnancy. These initial results were shared with WHO, which recommended that “IPTp with DP looks promising, but more research needed on its impact on adverse pregnancy outcomes”. This led to a the larger IMPROVE trial led by Dr. Hellen Barsosio from CGHR conducted in Kenya, Uganda and Malawi, again published in the Lancet. Surprisingly the investigator found that while DP was far superior in reducing malaria cases, SP remains more effective in preventing adverse pregnancy outcomes. This surprising finding was confirmed in 4 other trials conducted by other group in Africa. This paradox witnessed in IPTp-DP and IPTp-SPs trials, has led researchers to investigate SP’s potential non-malarial benefits, including its possible role in fetal growth promotion. These findings highlight the complexities in developing safe and effective interventions for malaria in pregnancy, as the search for alternatives continues through promising new avenues including malaria vaccines, monoclonal antibodies, and next-generation long-acting antimalarials.
In regard to post-discharge malaria chemoprevention (PDMC) for hospitalized children with severe anaemia, studies conducted in Kenya and Uganda led by Dr. Titus Kwambai and his team, revealed that children hospitalized with severe anemia face a high risk of death or readmission in the months following discharge.
A landmark clinical trial demonstrated that providing antimalarial drugs for 3 months post-discharge significantly reduced mortality and hospital readmissions for this highrisk group. WHO now recommends PDMC as a preventive treatment for children discharged from hospital with severe anaemia in areas of moderate to high malaria transmission in Africa

.
Despite significant progress, malaria remains a challenge with new studies showing how the mosquito is continuously adapting and the discovery of new species such as Anopheles stephensi. KEMRI researchers are now exploring the potential of malaria vaccines, monoclonal antibodies, and new drug combinations to further reduce the burden of the disease. With ongoing trials and global collaborations, KEMRI continues to play a critical role in shaping the future of malaria prevention and treatment.

